Biologics prescription for chronic inflammatory rheumatic diseases in Tunisia
##plugins.themes.academic_pro.article.main##
Abstract
Aims: To analyse the prescription of biologics (bDMARDs) in chronic inflammatory rheumatic diseases (CIRD) from Tunisian National Health Insurance (CNAM) data and to estimate their direct costs and associated factors.
Methods: One hundred and nine consecutive patients who received at least one bDMARDs during a six-month period from January to June 2022 were analysed. Clinical and therapeutic parameters as well as data related to the choice of bDMARDs were identified. Direct costs were assessed. Excess costs were considered if the monthly costs exceeded 2200 Tunisian dinars (TD) per patient.
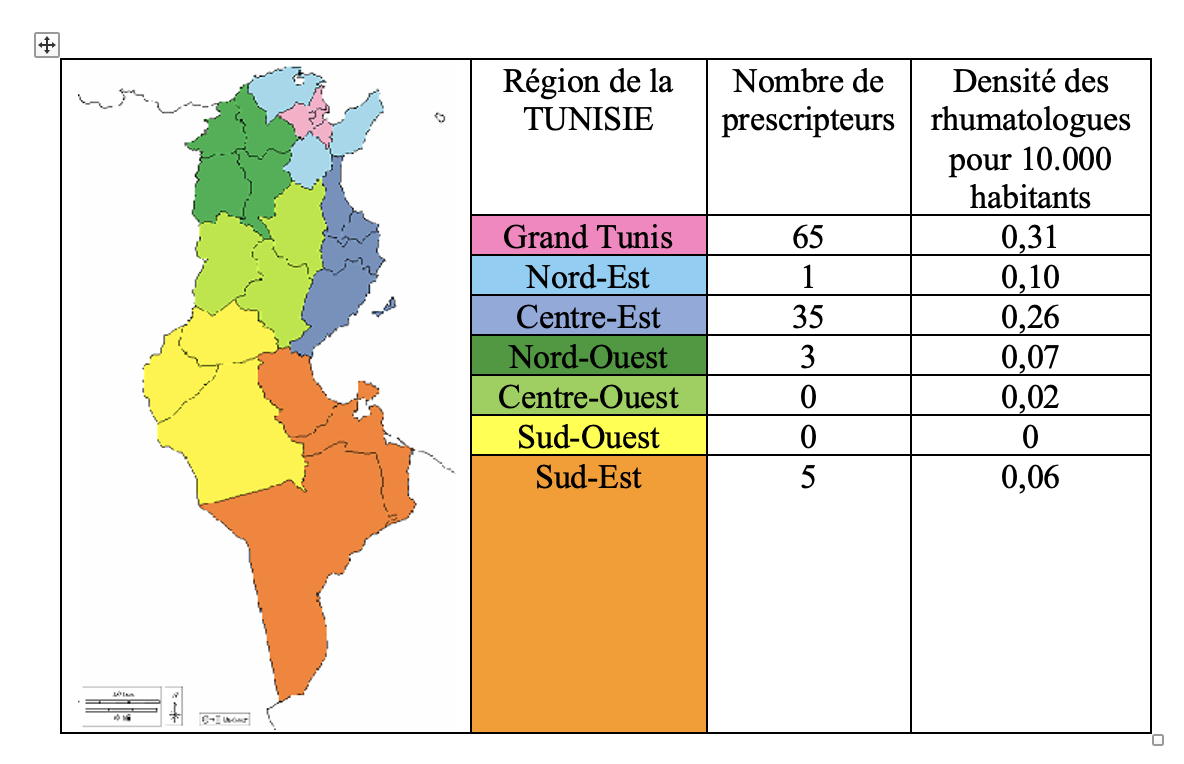
Results: The most common CIRD was axial spondylarthritis (AS) in 44% and rheumatoid arthritis (RA) in 37.6% of cases. Juvenile idiopathic arthritis represented 2.7% of cases. In Tunisia, prescribing of bDMARDs is concentrated in the coastal regions and follows the distribution of rheumatologists. Certolizumab pegol was the most prescribed agent in 45% of cases. No significant association was found between the choice of bDMARD and the characteristics of patients, CIRD or prescribers. The total monthly cost of bDMARDs was 225,535 ± 1269 TD. Overspending was significantly associated with initial high DAS28 in RA and young age and total hip replacement in AS.
Conclusion: Prescription of bDMARDs in CIRD is mainly for AS and in the coastal regions of Tunisia. The burden is considerable, partly due to the high cost of biologics. Data from this study may enable public health managers to better allocate the limited resources available for patient care and to develop medico-economic strategies to reduce health care costs.
Keywords:
Axial spondylarthritis , Rheumatoid arthritis, Health Insurance, Cost, Tumor Necrosis Factor Inhibitors, Molecular targeted therapy##plugins.themes.academic_pro.article.details##

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
References
- (1) Smolen J S. Insights into the treatment of rheumatoid arthritis: A paradigm in medicine. J Autoimmun 2020;110:102425.
- (2) Chen J, Wang X, Liu Y, Zhang X. Recent advances on neutrophil dysregulation in the pathogenesis of rheumatic diseases. Curr Opin Rheumatol 2024;36:142-7.
- (3) Atzeni F, Carriero A, Boccassini L, D'Angelo S. Anti-IL-17 Agents in the Treatment of Axial Spondyloarthritis. Immunotargets Ther 2021;10:141-153.
- (4) Listing J, Kekow J, Manger B et al. Mortality in rheumatoid arthritis: the impact of disease activity, treatment with glucocorticoids, TNFalpha inhibitors and rituximab.
- Ann Rheum Dis 2015;74:415-21.
- (5) Walsh J A, Magrey J M. Clinical Manifestations and Diagnosis of Axial Spondyloarthritis. Clin Rheumatol 2021;27:e547-60.
- (6) Stouten V, Pazmino S, Verschueren P et al. Comorbidity burden in the first three years after diagnosis in patients with rheumatoid arthritis, psoriatic arthritis or spondyloarthritis : a general practice registry-based study.
- RMD Open 2021;7:e001671.
- (7) Albrecht K, Regierer A C, Strangfeld A, Marschall U, Callhoff J. High burden of polypharmacy and comorbidity in persons with psoriatic arthritis: an analysis of claims data, stratified by age and sex. RMD Open 2023;9:e002960.
- (8) Schreiber S, Puig L, Gonçalves J, Mease PJ, Panaccione R, Emery P. Critical appraisal and future outlook on anti-inflammatory biosimilar use in chronic immune-mediated inflammatory diseases. Semin Arthritis Rheum 2022;55:152023.
- (9) Smolen JS, Landewé RBM, Bijlsma JWJ et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological diseasemodifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685‑99.
- (10) Fraenkel L, Bathon JM, England BR et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol 2021;73:1108‑23.
- (11) Wendling D, Hecquet S, Fogel O et al. 2022 French Society for Rheumatology (SFR) recommendations on the everyday management of patients with spondyloarthritis, including psoriatic arthritis. Joint Bone Spine 2022;89:105344.
- (12) van der Heijde D, Ramiro S, Landewe R et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017;76:978- 91.
- (13) Mahmoud I, Ben Tekaya A, Roueched L et al. Is improvement of fatigue in rheumatoid arthritis a proper effect of biologics? Rom J Intern Med. 2021 Mar 5;59(1):58-65. doi: 10.2478/rjim-2020-0028.
- (14) Fazaa A, Makhlouf Y, Ben Ouhiba A et al. Adherence to biologic disease-modifying antirheumatic drugs in adult patients with rheumatic diseases.
- Therapie. 2021 Sep-Oct;76(5):467-474. doi: 10.1016/j.therap.2020.08.003
- (15) Mahmoud I, Moalla M, Ben Tekaya A et al. Impact of FCGR2A R131H, FCGR3A F158V and FCGR3B NA1/NA2 polymorphisms on response to Fc-containing TNF inhibitors in Tunisian rheumatoid arthritis patients. Drug Metab Pers Ther. 2023 Mar 16;38(2):155-162. doi: 10.1515/dmpt-2022-0176.
- (16) Van der Woude D, van der Helm-van Mil AHM. Update on the epidemiology, factors, and disease outcomes of rheumatoid arthritis.
- Best Pract Res Clin Rheumtol 2018;32:174‑87
- (17) Hajjaji-Hassouni N. La polyarthrite rhumatoïde au Maroc: d'hier à d'aujourd'hui. Int J Med Surg 2017;4:41-4.
- (18) Claudepierre P, Fagnani F, Cukierman G et al. Le fardeau des spondyloarthrites graves en France : évaluation de la prévalence, des comorbidités et des coûts à l'échelle nationale. Rev Rhum 2019;86:490-6.
- (19) Almoallim H, Hassan R, Cheikh M et al. Rheumatoid Arthritis Saudi Database (RASD) : Disease characteristics and remission rates in a tertiary care center. Rheumatol Res Rev 2020; 12:139-45.
- (20) Mutlu MY, Tascilar K, Schett G. Rationnel, état des lieux et perspectives
- des combinaisons de traitements de fond biologiques dans la prise en charge de la polyarthrite rhumatoïde et du rhumatisme psoriasique. Rev Rhum 2023 ;90 :721-9
- (21) Ibfelt EH, Jensen DV, Hetland ML. The Danish nationwide clinical register for patients with rheumatoid arthritis: DANBIO. Clin Epidemiol 2016;8:737-42.
- (23) Yahya F, Gaffney K, Hamilton L et al. Tumour necrosis factor inhibitor survival and predictors of response in axial spondyloarthritis-findings from a United Kingdom cohort. Rheumatology 2018;57:619-24.
- (24) Maniscalco V, Maccora I, Girodo F et al. Anti-IL17 treatment in childhood chronic rheumatic diseases. Expert Opin Biol Ther. 2023;23:429-41.
- (25) Dougados M, Lucas J, Desfleurs E et al. Factors associated with the retention of secukinumab in patients with axial spondyloarthritis in real-world practice: results from a retrospective study (FORSYA). RMD Open 2023;9:e002802.
- (26) Vegas L P, Sbidian E, Wendling D et al. Factors associated with remission at 5-year follow-up in recent-onset axial spondyloarthritis: results from the DESIR cohort. Rheumatology 2022;61:1487–95
- (27) Vegas L P, Hoisnard L, Bastard L, Sbidian E, Claudepierre P. Long-term persistence of second-line biologics in psoriatic arthritis patients with prior TNF inhibitor exposure: a nationwide cohort study from the French health insurance database (SNDS). RMD Open 2022;8:e002681.
- (28) Dumaine C, Bekkar S, Belot A et al. Evénements indésirables infectieux chez des enfants atteints d'arthrite juvénile idiopathique et traités en vie réelle par biothérapies : données issues de la JIR cohorte. Rev Rhum 2021;88:443-9.
- (29) Cherif Chefchaouni A, Moutaouakkil Y, Mejdoub S et al. État des lieux de l'utilisation des anti-TNF α : adalimumab et étanercept chez les patients atteints de rhumatismes inflammatoires chroniques (RIC) dans une unité de pharmacie à l'hôpital. Pharm Hosp Clin 2021;56:193-200.
- (30) Lechat P. Médicaments biosimilaires : enjeux réglementaires et impacts médico-économiques. Bull Acad Natl Med 2020;204:877-83.
- (31) Freites-Núñez D, Baillet A, Rodriguez-Rodriguez L et al. Efficacy, safety and cost-effectiveness of a web-based platform delivering the results of a biomarker-based predictive model of biotherapy response for rheumatoid arthritis patients: a protocol for a randomized multicenter single-blind active controlled clinical trial (PREDIRA). Trials (2020) 21:755 https://doi.org/10.1186/s13063-020-04683-7
- (32) Stevenson M, Archer R, Tosh J et al. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: systematic review and economic evaluation. Health Technol Assess 2016;20:1-610.
- (33) Stevenson M D, Wailoo A J, Tosh J C et al. The Cost-effectiveness of Sequences of Biological Disease-modifying Antirheumatic Drug Treatment in England for Patients with Rheumatoid Arthritis Who Can Tolerate Methotrexate.
- J Rheumatol 2017;44:973-80.
- (34) Claxton L, Taylor M, Gerber R A et al. Modelling the cost-effectiveness of tofacitinib for the treatment of rheumatoid arthritis in the United States.
- Curr Med Res Opin 2018;34:1991-2000.
- (35) Gholami A, Azizpoor J, Aflaki E, Rezaee M, Keshavarz K. Cost-Effectiveness Analysis of Biopharmaceuticals for Treating Rheumatoid Arthritis: Infliximab, Adalimumab, and Etanercept. BioMed Research International 2021 ;4450162 : 12 p
- (36) Kuwana M, Tamura N, Yasuda S et al. Cost-effectiveness analyses of biologic and targeted synthetic disease-modifying anti-rheumatic diseases in patients with rheumatoid arthritis: Three approaches with a cohort simulation and real-world data. Mod Rheumatol 2023;33:302-11.
- (37) Erath A, Dusetzina S B. Assessment of Expected Out-of-Pocket Spending for Rheumatoid Arthritis Biologics Among Patients Enrolled in Medicare Part D, 2010-2019. JAMA Network Open 2020; 3: e203969.
- (38) Odes S, Greenberg D. A medicoeconomic review of early intervention with biologic agents in the treatment of inflammatory bowel diseases. ClinicoEconomics and Outcomes Research 2014;6:431–443.
- (39) Dong W, Hu X, Wu C et al. Efficacy, safety, and cost-effectiveness of therapeutic drug monitoring (TDM) for TNF inhibitor therapy in rheumatic disease: A systematic review and meta-analysis. Semin Arthritis Rheum 2023:63:152302.
- (40) van der Togt CJT, Van den Bemt B, Aletaha D, Alten R, Chatzidionysiou K, Galloway J. Points to consider for cost-effective use of biological and targeted synthetic DMARDs in inflammatory rheumatic diseases: results from an umbrella review and international Delphi study. RMD Open 2023;9:e002898.